Esters
Nomenclature
|
Formula
|
Functional
class name = alkyl alkanoate
Substituent suffix = -oate
|
|
- Esters are alkyl derivatives
of carboxylic acids.
- The easiest way to deal
with naming esters is to recognise the carboxylic acid and the alcohol that
they can be prepared from.
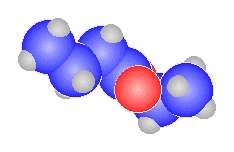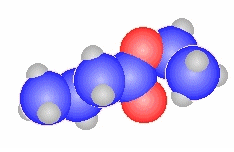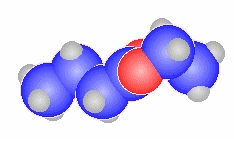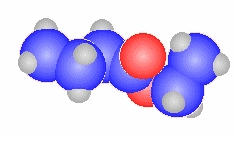
- The general ester, RCO2R'
can be derived from the carboxylic acid RCO2H and the alcohol HOR'
- The first component of
an ester name, the alkyl is derived from the
alcohol, R'OH.
- The second component
of an ester name, the -oate is derived from the
carboxylic acid, RCO2H.
- Alcohol
component
- the root name is based
on the longest chain containing the -OH group.
- The chain is numbered
so as to give the -OH the lowest possible number.
- Carboxylic
acid component
- the root name is based
on the longest chain including the carbonyl group.
- Since the carboxylic
acid group is at the end of the chain, it must be C1.
- The ester suffix for
the acid component is appended after the hydrocarbon suffix minus the "e"
: e.g. -ane + -oate = -anoate
etc.
- The complete ester name
is the alkyl alkanoate
- Functional group is an ester
- The alcohol component here is methanol, so the alkyl = methyl
- The acid component here is propanoic acid, so propanoate
methyl propanoate
|
CH3CH2C(=O)OCH3 |
|

Go to Main Menu
-
© M.EL-Fellah ,Chemistry
Department, Garyounis University
|