Amides
Nomenclature
|
Formula
|
Functional
class name = alkyl alkanamide
Substituent suffix = -amide
|
|
- Amides are amine derivatives
of carboxylic acids.
- The root name is based
on the longest chain including the carbonyl group of the amide group.
- Since the amide group
is at the end of the chain, the C=O carbon must be C1.
- The amide suffix is appended
after the hydrocarbon suffix minus the "e" : e.g.
-ane + -amide = -anamide etc.
- If the amide nitrogen
is substituted, the these substituents are given N- as the locant.
- The N- locant is listed
first.
- Functional group is an amide therefore suffix = -amide
- Hydrocarbon structure is an alkane therefore -an-
- The longest continuous chain is C4 therefore root = but
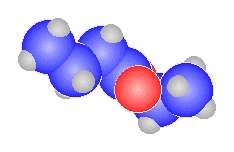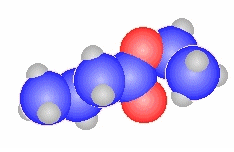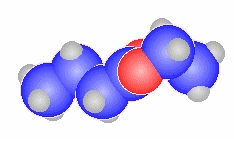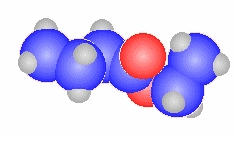
butanamide
|
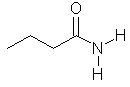
CH3CH2CH2C(=O)NH2 |
- Functional group is an amide therefore suffix = -amide
- Hydrocarbon structure is an alkane therefore -ane
- The longest continuous chain is C4 therefore root = but
- The nitrogen substituent is C1 i.e. an N-methyl group
N-methylbutanamide
|
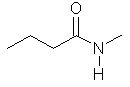
CH3CH2CH2C(=O)N(CH3)H |
- Functional group is an amide therefore suffix = -amide
- Hydrocarbon structure is an alkane therefore -ane
- The longest continuous chain is C2 therefore root = eth
- The two nitrogen substituents are C1 i.e. an N-methyl group
- There are two methyl groups, therefore multiplier = di-
N,N-dimethylethanamide
|
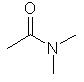
CH3C(=O)N(CH3)2 |

Go to Main Menu
-
© M.EL-Fellah ,Chemistry
Department, Garyounis University
|