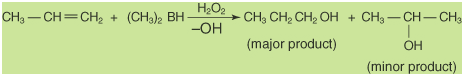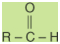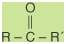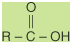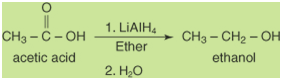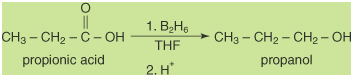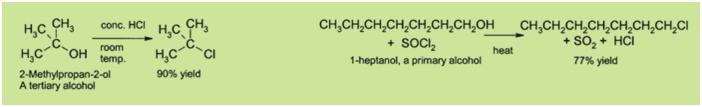|
Alcohol

Alcohols
is
any organic compound in which a hydroxyl group (-OH) is bound to a carbon
atom of an alkyl or substituted alkyl group. The general formula for a simple
acyclic alcohol is CnH2n+1OH. it usually refers to
ethanol, also known as grain alcohol or (older) spirits of wine,
or to any alcoholic beverage. Ethanol is a colorless, volatile liquid with a
mild odor which can be obtained by the fermentation of sugars. (Industrially, it
is more commonly obtained by ethylene hydration—the reaction of ethylene with
water in the presence of phosphoric acid.[1]) Ethanol is the most
widely used depressant in the world, and has been for thousands of years. This
sense underlies the term alcoholism (addiction to alcohol. Other alcohols are
usually described with a clarifying adjective, as in
isopropyl alcohol (propan-2-ol) or wood alcohol (methyl
alcohol, or methanol). The suffix -ol appears in the "official" IUPAC
chemical name of all alcohols.
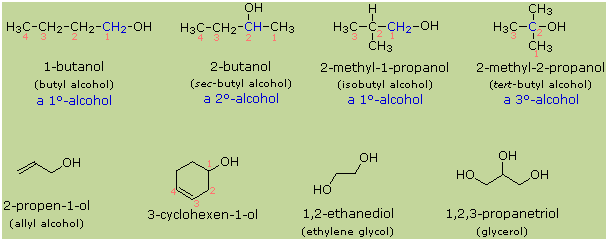
There are
three major subsets of alcohols: primary (1°), secondary (2°) and
tertiary (3°), based upon the number of carbon atoms the C-OH
group's carbon (shown in red) is bonded to. Ethanol is a simple 'primary'
alcohol. The simplest secondary alcohol is isopropyl alcohol (propan-2-ol), and
a simple tertiary alcohol is
tert-butyl alcohol (2-methylpropan-2-ol).
The phenols
with parent compound phenol have a hydroxyl group (attached to a benzene ring)
just like alcohols, but differ sufficiently in properties as to warrant a
separate treatment.
The simplest
and most commonly used alcohols are methanol and ethanol. Methanol was formerly
obtained by the distillation of wood and called "wood alcohol." It is now a
cheap commodity, the chemical product of carbon monoxide reacting with hydrogen
under high pressure. Methanol is intoxicating but not directly poisonous. It is
toxic by its breakdown (toxication) by the enzyme alcohol dehydrogenase in the
liver by forming formic acid and formaldehyde which cause permanent blindness by
destruction of the optic nerve.
Apart from
its familiar role in alcoholic beverages, ethanol is also used as a highly
controlled industrial solvent and raw material. To avoid the high taxes on
ethanol for consumption, additives are added to make it unpalatable (such as
denatonium benzoate—"Bitrex") or poisonous (such as methanol). Ethanol in this
form is known generally as denatured alcohol; when methanol is used, it may be
referred to as methylated spirits ("Meths") or "surgical spirits".
Two other
alcohols whose uses are relatively widespread (though not so much as those of
methanol and ethanol) are propanol and butanol. Like ethanol, they can be
produced by fermentation processes. (However, the fermenting agent is a
bacterium,
Clostridium acetobutylicum, that feeds on cellulose, not sugars like the
Saccharomyces yeast that produces ethanol.)
Nomenclature
In
the IUPAC system of nomenclature, functional groups are normally designated in
one of two ways. The presence of the function may be indicated by a
characteristic suffix and a location number. This is common for the
carbon-carbon double and triple bonds which have the respective suffixes ene
and yne. Halogens, on the other hand, do not have a suffix and are named
as substituents, for example: (CH3)2C=CHCHClCH3
is 4-chloro-2-methyl-2-pentene. If you are uncertain about the IUPAC rules for
nomenclature you should
review
them now.
Alcohols are usually named by the first procedure and are designated by an ol
suffix, as in ethanol, CH3CH2OH (note that a locator
number is not needed on a two-carbon chain). On longer chains the location of
the hydroxyl group determines chain numbering. For example: (CH3)2C=CHCH(OH)CH3
is 4-methyl-3-penten-2-ol. Other examples of IUPAC nomenclature are shown below,
together with the common names often used for some of the simpler compounds. For
the monofunctional alcohols, this common system consists of naming the alkyl
group followed by the word alcohol. Alcohols may also be classified
as primary, 1º, secondary, 2º & tertiary, 3º, in the same
manner as alkyl halides. This terminology refers to alkyl substitution of the
carbon atom bearing the hydroxyl group (colored blue in the illustration).
Many functional groups have a
characteristic
suffix
designator, and only one such suffix (other than "ene" and "yne") may be used in
a name. When the hydroxyl functional group is present together with a function
of higher
nomenclature
priority,
it must be cited and located by the prefix hydroxy and an appropriate
number. For example,
lactic acid
has the IUPAC name 2-hydroxypropanoic acid.
Compounds incorporating a C–S–H functional group are named thiols or
mercaptans. The IUPAC name of (CH3)3C–SH is
2-methyl-2-propanethiol, commonly called tert-butyl mercaptan. The chemistry of
thiols will not be described here, other than to note that they are stronger
acids and more powerful nucleophiles than alcohols.
Back
to the to
The hydroxyl group generally makes the
alcohol molecule polar. Those groups can form hydrogen bonds to
one another and to other compounds. This hydrogen bonding means
that alcohols can be used as protic solvents. Two opposing
solubility trends in alcohols are: the tendency of the polar OH
to promote solubility in water, and of the carbon chain to
resist it. Thus, methanol, ethanol, and propanol are miscible in
water because the hydroxyl group wins out over the short carbon
chain. Butanol, with a four-carbon chain, is moderately soluble
because of a balance between the two trends. Alcohols of five or
more carbons (Pentanol and higher) are effectively insoluble in
water because of the hydrocarbon chain's dominance. All simple
alcohols are miscible in organic solvents.
Because of hydrogen bonding, alcohols tend
to have higher boiling points than comparable hydrocarbons and
ethers. The boiling point of the alcohol ethanol is 78.29 °C,
compared to 69 °C for the hydrocarbon Hexane (a common
constituent of gasoline), and 34.6 °C for Diethyl ether.
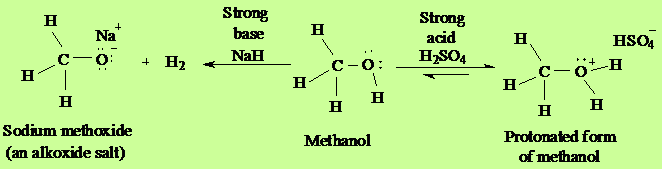
Alcohols, like water, can show either
acidic or basic properties at the O-H group. With a pKa
of around 16-19 they are generally slightly weaker acids than
water, but they are still able to react with strong bases such
as sodium hydride or reactive metals such as sodium. The salts
that result are called alkoxides, with the general
formula RO- M+.
Meanwhile the oxygen atom has lone pairs
of nonbonded electrons that render it weakly basic in the
presence of strong acids such as sulfuric acid. For example,
with methanol:
Preparation of Alcohols
can be prepared in a number of ways including the following: 1)Grignard
reaction. 2) Reduction of an aldehyde,
ketone, or carboxylic acid with the appropriate reducing agent.
3) Substitution reaction of hydroxide or water
on the appropriate alkyl halide. 4) Addition reactions
to alkenes (covered in the alkene section).
An alcohol preparation process is disclosed by catalytic hydrogenation of the
corresponding carbonyl compounds at high temperature and pressure in liquid
phase. A catalyst is used that contains copper on a SiO2-containing substrate in
the presence or absence of one or several elements selected among magnesium,
barium, zinc or chromium.
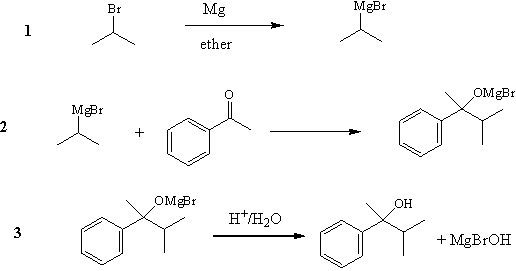
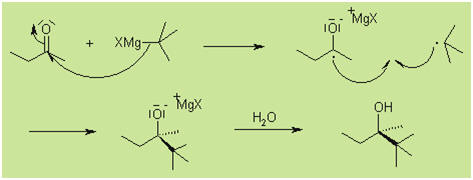
The Grignard reaction
is one of the most important carbon-carbon bond forming reactions in organic
chemistry.
The Grignard Reaction is the addition of an
organomagnesium halide (Grignard reagent) to a ketone or aldehyde, to form a
tertiary or secondary alcohol, respectively. The reaction with formaldehyde
leads to a primary alcohol.
Grignard Reagents are also used in the following
important reactions: The addition of an excess of a Grignard reagent to an
ester or lactone gives a tertiary alcohol in which two alkyl groups are the
same, and the addition of a Grignard reagent to a nitrile produces an
unsymmetrical ketone via a metalloimine intermediate. (Some more reactions
are depicted below)
Possible alkyl
bromides:
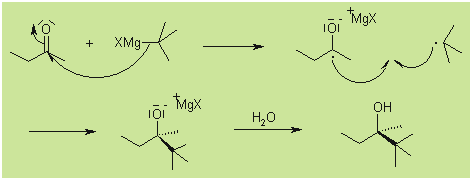
Mechanism
While the reaction is generally thought to proceed
through a nucleophilic addition mechanism, sterically hindered substrates
may react according to an SET (single electron transfer) mechanism:
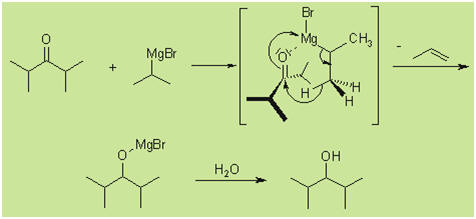
With sterically hindered ketones the following side
products are received:
The Grignard reagent can act as base, with
deprotonation yielding an enolate intermediate. After work up, the starting
ketone is recovered.
A reduction can also take place, in which a hydride is
delivered from the β-carbon of the Grignard reagent to the carbonyl carbon
via a cyclic six-membered transition state.
Additional Reactions of Grignard
Reagents:
With carboxylic acid chlorides:
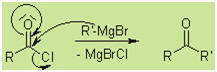
Esters are less reactive than the intermediate
ketones, therefore the reaction is only suitable for synthesis of tertiary
alcohols using an excess of Grignard Reagent:

With nitriles:
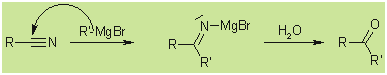
With CO2 (by adding dry ice to the reaction
mixture):
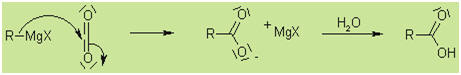
With oxiranes:

Back
to the to
2)
Reduction
Alcohols can be prepared by the
hydration of alkenes or by the reduction of aldehydes,
ketones, acids, and esters.
The elements of water can be added to
the double-bonded carbons of an alkene in either a
Markovnikov's or an anti-Markovnikov's manner. As shown in
the following figure, a hydrogen ion catalyzes the
Markovnikov's addition.
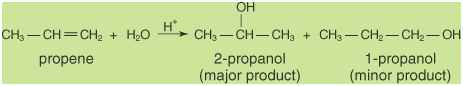
The anti-Markovnikov's addition results
from a hydroboration-oxidation reaction.
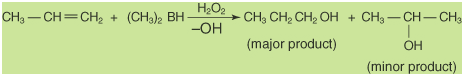
You can find the mechanisms for both the
Markovnikov's and anti-Markovnikov's addition of water in
CliffsQuickReview Organic Chemistry I.
An aldehyde has a structural formula
of
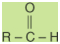
while the structural formula of a
ketone is 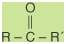
In these formulas, the R or R′ group
may be either an aliphatic or aromatic group. In a ketone,
the R and R′ groups may represent the same group or
different groups. These types of compounds are best reduced
by complex metal hydrides, such as lithium aluminum hydride
(LiAlH4) or sodium borohydride (NaBH4).
Following are two examples of complex
metal reductions:
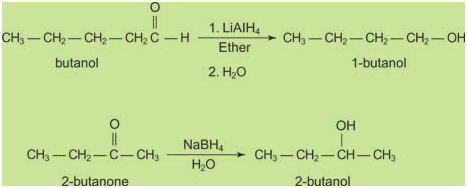
Lithium aluminum hydride is a very
strong reducing agent that will reduce many functional
groups in addition to aldehydes and ketones. Sodium
borohydride is a much weaker reducing agent that basically
will reduce only aldehydes and ketones to alcohols.
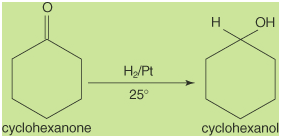
You can also catalytically reduce
aldehydes and ketones to produce 1° and 2° alcohols.
Reduction conditions are very similar to those used to
reduce alkene double bonds. If a molecule possesses both a
double bond and an aldehyde or ketone functional group,
reduction of the aldehyde or ketone group is best carried
out using sodium borohydride. The reduction of cyclohexanone
by hydrogen gas with a platinum catalyst produces
cyclohexanol in good yield.
Reduction of carboxylic acids
The reduction of a carboxylic acid:
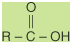
leads to the formation of
a primary alcohol:

This reduction requires a
very strong reducing agent, and lithium aluminum hydride is
the standard choice.
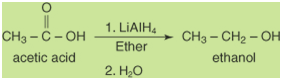
Diborane, B2H6,
also reduces carboxylic acids to alcohols.
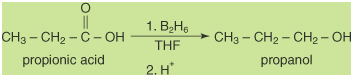
Catalytic hydrogenation
gives very poor yields and is not usually used for this type
of reaction.
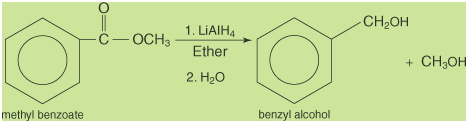
Esters, like carboxylic acids, are
normally reduced with lithium aluminum hydride. In these
reactions, two alcohols are formed. An example is the
reduction of methyl benzoate to benzyl alcohol and methanol.
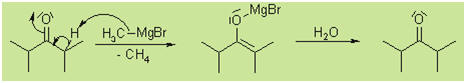
Grignard reaction with aldehydes
and ketones
The Grignard reaction is
the only simple method available that is capable of
producing primary, secondary, and tertiary alcohols. To
produce a primary alcohol, the Grignard reagent is reacted
with formaldehyde.
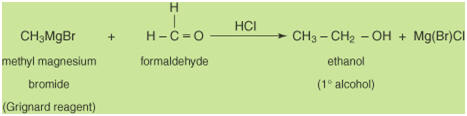
Reacting a Grignard reagent with any other aldehyde will
lead to a secondary alcohol.
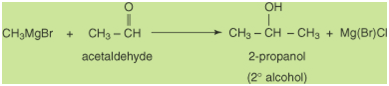
Finally, reacting a
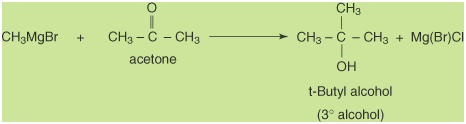
Grignard reagent with a ketone will generate a tertiary
alcohol.
Back
to the to
|
Reactions of Alcohols
1.
Substitution of the Hydroxyl Hydrogen
Alcohols can behave as
weak acids, undergoing deprotonation. The deprotonation reaction
to produce an alkoxide salt is either performed with a strong
base such as sodium hydride or n-butyllithium, or with
sodium or potassium metal.
-
2 R-OH + 2 NaH → 2 R-O-Na+
+ 2H2↑
-
2 R-OH + 2Na → 2R-O−Na
+ H2
-
E.g. 2 CH3CH2-OH
+ 2 Na → 2 CH3-CH2-O−Na + H2
Water is similar in pKa
to many alcohols, so with sodium hydroxide there is an
equilibrium set up which usually lies to the left:
-
R-OH + NaOH <=> R-O-Na+
+ H2O (equilibrium to the left)
It should be noted,
though, that the bases used to deprotonate alcohols are strong
themselves. The bases used and the alkoxides created are both
highly moisture sensitive chemical reagents.
The acidity of alcohols is
also affected by the overall stability of the alkoxide ion.
Electron-withdrawing groups attached to the carbon containing
the hydroxyl group will serve to stabilize the alkoxide when
formed, thus resulting in greater acidity. On the other hand,
the presence of electron-donating group will result in a less
stable alkoxide ion formed. This will result in a scenario
whereby the unstable alkoxide ion formed will tend to accept a
proton to reform the original alcohol.
With alkyl halides
alkoxides give rise to ethers in the Williamson ether synthesis.
Because of its enhanced acidity, the hydrogen atom on the hydroxyl group is
rather easily replaced by other substituents. A simple example is the facile
reaction of simple alcohols with sodium (and sodium hydride), as described in
the first equation below. Another such substitution reaction is the isotopic
exchange that occurs on mixing an alcohol with deuterium oxide (heavy water).
This exchange, which is catalyzed by acid or base, is very fast under normal
conditions, since it is difficult to avoid traces of such catalysts in most
experimental systems.
|
2 R–O–H + 2 Na
2 R–O(–)Na(+) + H2 |
|
R–O–H
+
D2O
R–O–D
+
D–O–H |
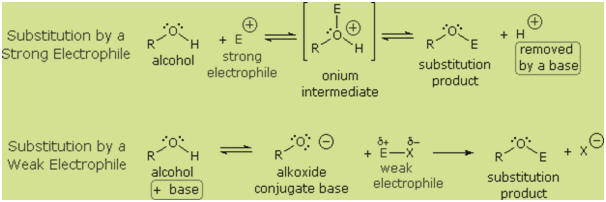
The
mechanism by which many substitution reactions of this kind take place is
straightforward. The oxygen atom of an alcohol is nucleophilic and is therefore
prone to attack by electrophiles. The resulting "onium" intermediate then loses
a proton to a base, giving the substitution product. If a strong electrophile is
not present, the nucleophilicity of the oxygen may be enhanced by conversion to
its conjugate base (an alkoxide). This powerful nucleophile then attacks the
weak electrophile. These two variations of the substitution mechanism are
illustrated in the following diagram.
The
preparation of tert-butyl hypochlorite from tert-butyl alcohol is an example of
electrophilic halogenation of oxygen, but this reaction is restricted to
3º-alcohols because 1º and 2º-hypochlorites lose HCl to give aldehydes and
ketones. In the following equation the electrophile may be regarded as Cl(+).
(CH3)3C–O–H
+
Cl2
+ NaOH (CH3)3C–O–Cl
+ NaCl
+ H2O
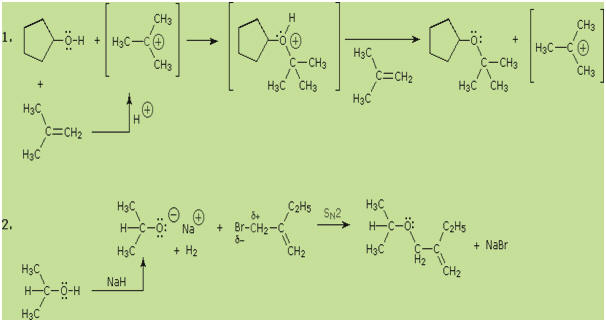
Alkyl substitution of the hydroxyl group leads to ethers. This reaction provides
examples of both strong electrophilic substitution (first equation below), and
weak electrophilic substitution (second equation). The latter SN2
reaction is known as the Williamson Ether Synthesis, and is generally
used only with 1º-alkyl halide reactants because the strong alkoxide base leads
to E2 elimination of 2º and 3º-alkyl halides.
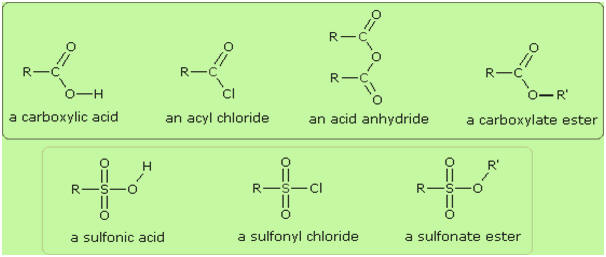
One
of the most important substitution reactions at oxygen is ester formation
resulting from the reaction of alcohols with electrophilic derivatives of
carboxylic and sulfonic acids. The following illustration displays the general
formulas of these reagents and their ester products, in which the R'–O– group
represents the alcohol moiety. The electrophilic atom in the acid chlorides and
anhydrides is colored red. Examples of specific esterification reactions may be
selected from the menu below the diagram, and will be displayed in the same
space.
To form an ester from an alcohol and a
carboxylic acid the reaction, known as Fischer esterification,
is usually performed at reflux with a catalyst of concentrated
sulfuric acid:
- R-OH + R'-COOH → R'-COOR + H2O
In order to drive the equilibrium to the
right and produce a good yield of ester, water is usually
removed, either by an excess of H2SO4 or
by using a Dean-Stark apparatus. Esters may also be prepared by
reaction of the alcohol with an acid chloride in the presence of
a base such as pyridine.
Other types of ester are prepared
similarly- for example tosyl (tosylate) esters are made by
reaction of the alcohol with p-toluenesulfonyl chloride in
pyridine.
2.
Nucleophilic Substitution of the Hydroxyl Group
The OH group is not a good leaving group
in nucleophilic substitution reactions, so neutral alcohols do
not react in such reactions. However, if the oxygen is first
protonated to give R−OH2+, the leaving
group (water) is much more stable, and the nucleophilic
substitution can take place. For instance, tertiary alcohols
react with hydrochloric acid to produce tertiary alkyl halides,
where the hydroxyl group is replaced by a chlorine atom by
unimolecular nucleophilic substitution. If primary or secondary
alcohols are to be reacted with hydrochloric acid, an activator
such as zinc chloride is needed. Alternatively the conversion
may be performed directly using thionyl chloride.
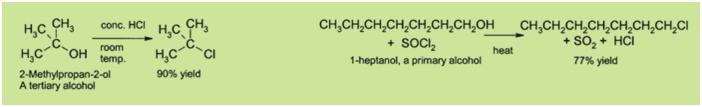
Alcohols may likewise be converted to
alkyl bromides using hydrobromic acid or phosphorus tribromide,
for example:
- 3 R-OH + PBr3 → 3 RBr + H3PO3
In the Barton-McCombie deoxygenation an
alcohol is deoxygenated to an alkane with tributyltin hydride or
a trimethylborane-water complex in a radical substitution
reaction.
Using the chemical behavior of alkyl halides as a reference, we are encouraged
to look for analogous substitution and elimination reactions of alcohols. The
chief difference, of course, is a change in the leaving anion from halide to
hydroxide. Since oxygen is slightly more electronegative than chlorine (3.5 vs.
2.8 on the Pauling scale), we expect the C-O bond to be more polar than a C-Cl
bond. Furthermore, an independent measure of the electrophilic character of
carbon atoms from their nmr chemical shifts (both 13C & alpha
protons), indicates that oxygen and chlorine substituents exert a similar
electron-withdrawing influence when bonded to sp3 hybridized carbon
atoms. Despite this promising background evidence, alcohols do not undergo the
same SN2 reactions commonly observed with alkyl halides. For example,
the rapid SN2 reaction of 1-bromobutane with sodium cyanide, shown
below, has no parallel when 1-butanol is treated with sodium cyanide. In fact
ethyl alcohol is often used as a solvent for alkyl halide substitution reactions
such as this.
|
CH3CH2CH2CH2–Br +
Na(+)
CN(–)
CH3CH2CH2CH2–CN +
Na(+)
Br(–) |
|
CH3CH2CH2CH2–OH +
Na(+)
CN(–)
No Reaction |
The
key factor here is the stability of the leaving anion (bromide vs. hydroxide).
We know that HBr is a much stronger acid than water (by more than 18 powers of
ten), and this difference will be reflected in reactions that generate their
conjugate bases. The weaker base, bromide anion, is more stable and its release
in a substitution or elimination reaction will be much more favorable than that
of hydroxide ion, a stronger and less stable base.
Clearly, an obvious step toward improving the reactivity of alcohols in SN2
reactions would be to modify the –OH functional group in a way that improves its
stability as a leaving anion. One such modification is to conduct the
substitution reaction in strong acid so that –OH is converted to –OH2(+).
Since the hydronium ion (H3O(+)) is a much stronger acid
than water, its conjugate base (H2O) is a better leaving group than
hydroxide ion. The only problem with this strategy is that many nucleophiles,
including cyanide, are deactivated by protonation in strong acid, effectively
removing the nucleophilic co-reactant needed for the substitution. The strong
acids HCl, HBr and HI are not subject to this difficulty because their conjugate
bases are good nucleophiles and are even weaker bases than alcohols. The
following equations illustrate some substitution reactions of alcohols that may
be effected by these acids. As was true for alkyl halides, nucleophilic
substitution of 1º-alcohols proceeds by a SN2 mechanism, whereas
3º-alcohols react by a SN1 mechanism. Reactions of 2º-alcohols may
occur by both mechanisms and often produce some rearranged products. The numbers
in parentheses next to the mineral acid formulas represent the weight percentage
of a concentrated aqueous solution, the form in which these acids are normally
used.
|
CH3CH2CH2CH2–OH +
HBr
(48%)
CH3CH2CH2CH2–OH2(+)
Br(–)
CH3CH2CH2CH2–Br
+ H2O
SN2
|
|
(CH3)3C–OH + HCl
(37%)
(CH3)3C–OH2(+)
Cl(–)
(CH3)3C(+)
Cl(–)
+ H2O (CH3)3C–Cl
+ H2O
SN1 |
Although these reactions are sometimes referred to as "acid-catalyzed" this is
not strictly correct. In the overall transformation a strong HX acid is
converted to water, a very weak acid, so at least a stoichiometric quantity of
HX is required for a complete conversion of alcohol to alkyl halide. The
necessity of using equivalent quantities of very strong acids in this reaction
limits its usefulness to simple alcohols of the kind shown above. Alcohols
having acid sensitive groups would, of course, not tolerate such treatment.
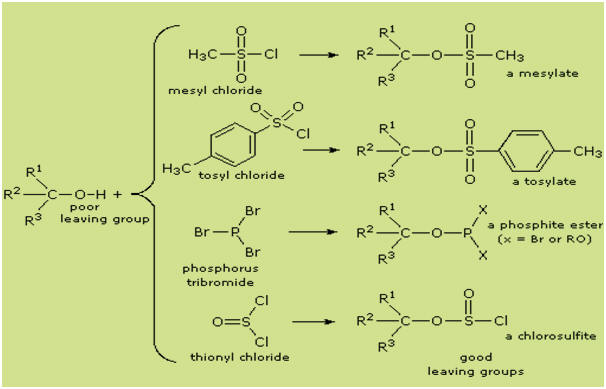
Nevertheless, the idea of modifying the -OH functional group to improve its
stability as a leaving anion can be pursued in other directions. The following
diagram shows some modifications that have proven effective. In each case the
hydroxyl group is converted to an ester of a strong acid. The first two examples
show the sulfonate esters described earlier. The third and fourth examples show
the formation of a phosphite ester (X represents remaining bromines or
additional alcohol substituents) and a chlorosulfite ester respectively. All of
these leaving groups (colored blue) have conjugate acids that are much stronger
than water (by 13 to 16 powers of ten) so the leaving anion is correspondingly
more stable than hydroxide ion. The mesylate and tosylate compounds are
particularly useful in that they may be used in substitution reactions with a
wide variety of nucleophiles. The intermediates produced in reactions of
alcohols with phosphorus tribromide and thionyl chloride (last two examples) are
seldom isolated, and these reactions continue on to alkyl bromide and chloride
products.
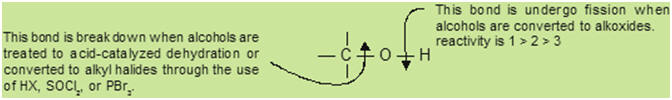
The
importance of sulfonate ester intermediates in general nucleophilic substitution
reactions of alcohols may be illustrated by the following conversion of
1-butanol to pentanenitrile (butyl cyanide), a reaction that does not occur with
the alcohol alone (see
above).
The phosphorus and thionyl halides, on the other hand, only act to convert
alcohols to the corresponding alkyl halides.
|
CH3CH2CH2CH2–OH +
CH3SO2Cl |
pyridine
|
CH3CH2CH2CH2–OSO2CH3 |
Na(+)
CN(–)
|
CH3CH2CH2CH2–CN
+
CH3SO2O(–)
Na(+) |
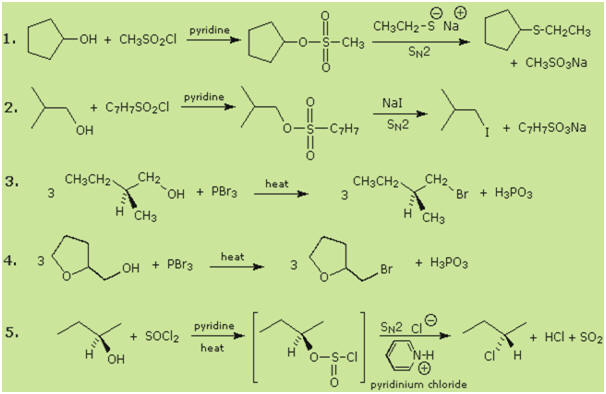
Some examples of alcohol substitution reactions using this approach to
activating the hydroxyl group are shown in the following diagram. The first two
cases serve to reinforce the fact that sulfonate ester derivatives of alcohols
may replace alkyl halides in a variety of SN2 reactions. The next two
cases demonstrate the use of phosphorus tribromide in converting alcohols to
bromides. This reagent may be used without added base (e.g. pyridine), because
the phosphorous acid product is a weaker acid than HBr. Phosphorous tribromide
is best used with 1º-alcohols, since 2º-alcohols often give rearrangement
by-products resulting from competing SN1 reactions. Note that the
ether oxygen in reaction 4 is not affected by this reagent; whereas, the
alternative synthesis using concentrated HBr cleaves ethers. Phosphorus
trichloride (PCl3) converts alcohols to alkyl chlorides in a similar
manner, but thionyl chloride is usually preferred for this transformation since
the inorganic products are gases (SO2 & HCl). Phosphorus triiodide is
not stable, but may be generated in situ from a mixture of red phosphorus
and bromine, and acts to convert alcohols to alkyl iodides. The last example
shows the reaction of thionyl chloride with a chiral 2º-alcohol. The presence of
an organic base such as pyridine is important, because it provides a substantial
concentration of chloride ion needed for the final SN2 reaction of
the chlorosufite intermediate. In the absence of base chlorosufites decompose on
heating to give the expected alkyl chloride with retention of configuration
Tertiary alcohols are not commonly used for substitution reactions of the kind
discussed here, because SN1 and E1 reaction paths are dominant and
are difficult to control. This aspect of alcohol chemistry will be touched upon
in the next section.
The
importance of sulfonate esters as intermediates in many substitution reactions
cannot be overstated. A rigorous proof of the configurational inversion that
occurs at the substitution site in SN2 reactions makes use of such
reactions. An example of such a proof will display above when the
An
Inversion Proof
button beneath the diagram is pressed. Abbreviations for the more commonly used
sulfonyl derivatives are given in the following table.
|
Sulfonyl Group |
CH3SO2– |
CH3C6H4SO2– |
BrC6H4SO2– |
CF3SO2– |
|
Name & Abbrev. |
Mesyl or Ms |
Tosyl or Ts |
Brosyl or Bs |
Trifyl or Tf |
Back
to the to
3.
Elimination Reactions of Alcohols
In
the discussion of alkyl halide reactions we noted that 2º and 3º-alkyl halides
experienced rapid
E2 elimination
when treated with strong bases, such as hydroxide and alkoxides. Alcohols do not
undergo such base-induced elimination reactions and are, in fact, often used as
solvents for such reactions. This is yet another example of how leaving group
stability often influences the rate of a reaction.
When an alcohol is treated with sodium hydroxide, the following acid-base
equilibrium occurs. Most alcohols are slightly weaker acids than water so the
left side is favored.
R–O–H
+ Na(+) OH(–)
R–O(–) Na(+) +
H–OH
The
elimination of water from an alcohol is called dehydration. Recalling
that water is a much better leaving group than hydroxide ion, it is sensible to
use acid-catalysis rather than base-catalysis to achieve such reactions. Four
examples of this useful technique are shown below. Note that hydrohalic acids
(HX) are not normally used as catalysts because their conjugate bases are good
nucleophiles and may give
substitution
products.
The conjugate bases of sulfuric and phosphoric acids are not good nucleophiles
and do not give substitution under the usual conditions of their use.
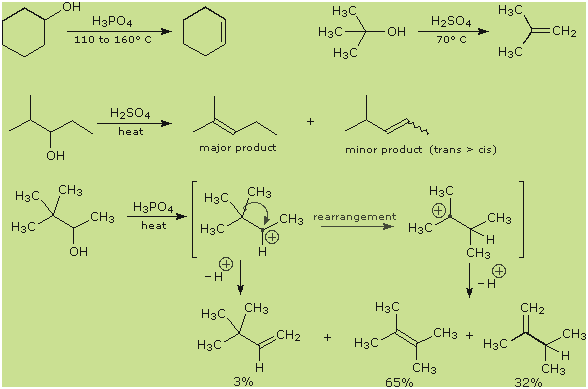
The
first two examples (top row) are typical, and the more facile elimination of the
3º-alcohol suggests predominant
E1 character
for the reaction. This agrees with the tendency of branched 1º and 2º-alcohols
to give rearrangement products, as shown in the last example. The last two
reactions also demonstrate that the
Zaitsev Rule
applies to alcohol dehydrations as well as alkyl halide eliminations. Thus the
more highly-substituted double bond isomer is favored among the products.
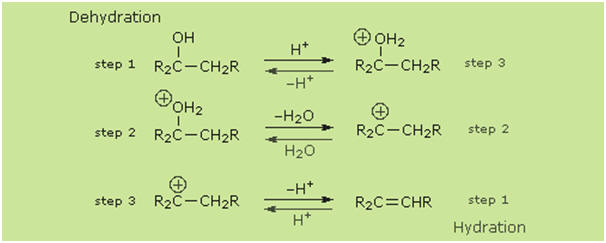
It should be noted that the acid-catalyzed dehydrations discussed here are the
reverse of the
acid-catalyzed
hydration reactions of alkenes.
Indeed, for reversable reactions such as this the laws of thermodynamics require
that the mechanism in both directions proceed by the same reaction path. This is
known as the principle of microscopic reversibility. To illustrate, the
following diagram lists the three steps in each transformation. The dehydration
reaction is shown by the blue arrows; the hydration reaction by magenta arrows.
The intermediates in these reactions are common to both, and common transition
states are involved. This can be seen clearly in the energy diagrams depicted by
clicking the button beneath the equations.
More useful is the E1 elimination reaction
of alcohols to produce alkenes. The reaction generally obeys
Zaitsev's Rule, which states that the most stable (usually the
most substituted) alkene is formed. Tertiary alcohols eliminate
easily at just above room temperature, but primary alcohols
require a higher temperature.
This is a diagram of acid catalysed
dehydration of ethanol to produce ethene:

A more controlled elimination reaction is
the Chugaev elimination with carbon disulfide and iodomethane.Base
induced E2 eliminations of alcohols may be achieved if their
sulfonate ester derivatives are used. This has the advantage of
avoiding strong acids, which may cause molecular rearrangement
and / or double bond migration in some cases. Since 3º-sulfonate
derivatives are sometimes unstable, this procedure is best used
with 1º and 2º-mesylates or tosylates. Application of this
reaction sequence is shown here for 2-butanol. The Zaitsev Rule
favors formation of 2-butene (cis + trans) over 1-butene.
|
CH3CH2CH(CH3)–OH |
CH3SO2Cl
|
CH3CH2CH(CH3)–OSO2CH3
|
C2H5O(–)Na(+)
|
CH3CH=CHCH3 + CH3CH2CH=CH2
+
CH3SO2O(–)
Na(+) +
C2H5OH |
The
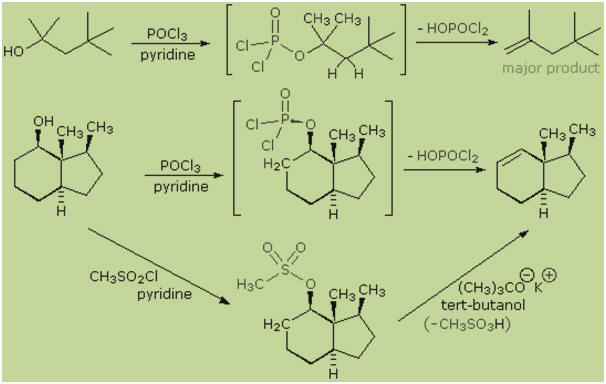
E2 elimination of 3º-alcohols under relatvely non-acidic conditions may be
accomplished by treatment with phosphorous oxychloride (POCl3) in
pyridine. This procedure is also effective with hindered 2º-alcohols, but for
unhindered and 1º-alcohols an SN2 chloride ion substitution of the
chlorophosphate intermediate competes with elimination. Some examples of these
and related reactions are given in the following figure. The first equation
shows the dehydration of a 3º-alcohol. The predominance of the non-Zaitsev
product (less substituted double bond) is presumed due to steric hindrance of
the methylene group hydrogens, which interferes with the approach of base at
that site. The second example shows two elimination procedures applied to the
same 2º-alcohol. The first uses the single step POCl3 method, which
works well in this case because SN2 substitution is retarded by
steric hindrance. The second method is another example in which an intermediate
sulfonate ester confers halogen-like reactivity on an alcohol. In every case the
anionic leaving group is the conjugate base of a strong acid.
Back
to the to
-
© M.EL-Fellah ,Chemistry
Department, Garyounis University
|