|
Basic IUPAC Organic Nomenclature
| |||||||||||||||
Acyl
Halides or Acid Halides
|
| Nomenclature |
Formula
|
3D structure
|
| Functional
class name = acyl or acid halide Substituent suffix = -oyl halide |
 |
|
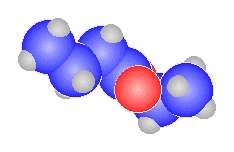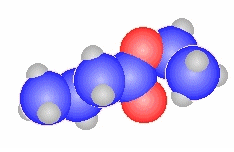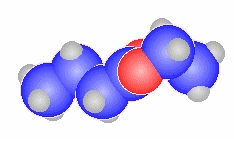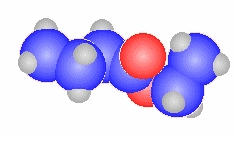
ethanoyl chloride
|
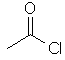 CH3C(=O)Cl |
|
butanoyl chloride
|
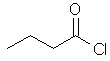 CH3CH2CH2C(=O)Cl |
|
2-methylpropanoyl chloride
|
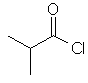 (CH3)2CHC(=O)Cl |
|

Go to Main Menu
© M.EL-Fellah ,Chemistry Department, Garyounis University