|
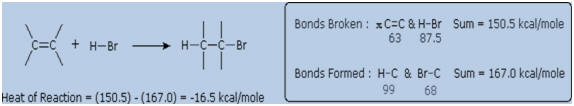
The most common chemical transformation of a carbon-carbon double
bond is the addition reaction. A large number of reagents, both
inorganic and organic, have been found to add to this functional group,
and in this section we shall review many of these reactions. A majority
of these reactions are
exothermic, due to the fact that the C-C pi-bond is
relatively weak (ca. 63 kcal/mole) relative to the sigma-bonds formed to
the atoms or groups of the reagent. Remember, the bond energies of a
molecule are the energies required to break (homolytically) all the
covalent bonds in the molecule. Consequently, if the bond energies of
the product molecules are greater than the bond energies of the
reactants, the reaction will be exothermic. The following calculations
for the addition of H-Br are typical. Note that by convention
exothermic reactions have a negative heat of reaction.
1. Addition of Strong Brønsted
Acids
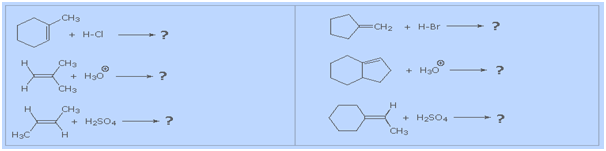
As illustrated by the preceding general equation, strong Brønsted
acids such as HCl, HBr, HI & H2SO4,
rapidly add to the C=C functional group of alkenes to give products in
which new covalent bonds are formed to hydrogen and to the conjugate
base of the acid. Using the above equation as a guide, write the
addition products expected on reacting each of these reagents with
cyclohexene.
Weak Brønsted acids such as water (pKa
= 15.7) and acetic acid (pKa
= 4.75) do not normally add to alkenes. However, the addition of a
strong acid serves to catalyze the addition of water, and in this way
alcohols may be prepared from alkenes. For example, if sulfuric acid is
dissolved in water it is completely ionized to the hydronium ion, H3O(+),
and this strongly acidic (pKa
= -1.74) species effects hydration of ethene and other alkenes.
CH2=CH2
+ H3O(+)
——>
HCH2–CH2OH
+ H(+)
The importance of choosing an appropriate solvent for these
addition reactions should now be clear. If the addition of HCl, HBr or
HI is desired, water and alcohols should not be used. These strong acids
will ionize in such solvents to give ROH2(+)
and the nucleophilic oxygen of the solvent will compete with the halide
anions in the final step, giving alcohol and ether products. By using
inert solvents such as hexane, benzene and methylene chloride, these
competing solvent aditions are avoided. Because these additions proceed
by way of polar or ionic intermediates, the rate of reaction is greater
in polar solvents, such as nitromethane and acetonitrile, than in
non-polar solvents, such as cyclohexane and carbon tetrachloride.
Regioselectivity and the
Markovnikov Rule
Only one product is possible from the addition of these strong
acids to symmetrical alkenes such as ethene and cyclohexene. However, if
the double bond carbon atoms are not structurally equivalent, as in
molecules of 1-butene, 2-methyl-2-butene and 1-methylcyclohexene, the
reagent conceivably may add in two different ways. This is shown for
2-methyl-2-butene in the following equation.
|
(CH3)2C=CHCH3 +
H-Cl |
 |
(CH3)2CH–CHClCH3 |
or |
(CH3)2CCl–CHHCH3 |
|
2-methyl-2-butene |
|
2-chloro-3-methylbutane |
|
2-chloro-2-methylbutane |
When addition reactions to such unsymmetrical alkenes are carried
out, we find that one of the two possible constitutionally isomeric
products is formed preferentially. Selectivity of this sort is termed
regioselectivity. In the above example, 2-chloro-2-methylbutane is
nearly the exclusive product. Similarly, 1-butene forms 2-bromobutane as
the predominant product on treatment with HBr.
After studying many addition reactions of this kind, the Russian
chemist Vladimir Markovnikov noticed a trend in the structure of the
favored addition product. He formulated this trend as an empirical rule
we now call
The Markovnikov Rule: When a Brønsted acid, HX, adds to an unsymmetrically substituted
double bond, the acidic hydrogen of the acid bonds to that carbon of the
double bond that has the greater number of hydrogen atoms already
attached to it.
In more homelier vernacular this rule may be restated as, "Them that
has gits."
|
It is a helpful exercise
to predict the favored product in examples such as those
shown below: |
|
 |
Empirical rules like the Markovnikov Rule are useful aids for remembering and
predicting experimental results. Indeed, empirical rules are often the
first step toward practical mastery of a subject, but they seldom
constitute true understanding. The Markovnikov Rule, for example,
suggests there are common and important principles at work in these
addition reactions, but it does not tell us what they are. The next step
in achieving an understanding of this reaction must be to construct a
rational mechanistic model that can be tested by
experiment.
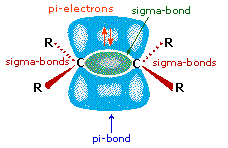
All the reagents discussed
here are strong Brønsted acids so, as a first step, it seems sensible to
find a base with which the acid can react. Since we know that these
acids do not react with alkanes, it must be the pi-electrons of the
alkene double bond that serve as the base. As shown in the diagram on
the right, the pi-orbital extends into the space immediately above and
below the plane of the double bond, and the electrons occupying this
orbital may be attracted to the proton of a Brønsted acid. The resulting
acid-base equilibrium generates a carbocation intermediate (the
conjugate acid of the alkene) which then combines rapidly with the
anionic conjugate base of the Brønsted acid. This two-step mechanism is
illustrated for the reaction of ethene with hydrogen chloride by the
following equations.
|
First
Step: H2C=CH2 +
HCl
|
 |
HH2C=CH2(+) + Cl(–) |
|
Second Step: HH2C=CH2(+) +
Cl(–)
|
 |
HH2C=CH2Cl |
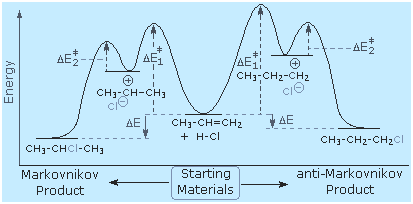
An energy diagram for this two-step addition mechanism is shown to
the left. From this diagram we see that the slow or rate-determining
step (the first step) is also the product determining step (the anion
will necessarily bond to the carbocation site). Electron donating double
bond substituents increase the reactivity of an alkene, as evidenced by
the increased rate of hydration of 2-methylpropene (two alkyl groups)
compared with 1-butene (one alkyl group). Evidently, alkyl substituents
act to increase the rate of addition by lowering the activation energy,
ΔE‡1
of the rate determining step, and it is here we should look for a
rationalization of Markovnikov's rule.
As expected, electron withdrawing substituents, such as fluorine or
chlorine, reduce the reactivity of an alkene to addition by acids (vinyl
chloride is less rective than ethene).
|
First Step:
H2C=CH2
+
HCl
|
 |
HH2C–CH2(+)
+
Cl(–) |
| Second Step:
HH2C–CH2(+)
+
Cl(–)
|
 |
HH2C–CH2Cl |
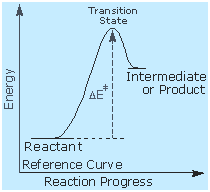
George Hammond formulated a useful principle that relates the
nature of a transition state to its location on the reaction path. This
Hammond Postulate states that
a transition state will be
structurally and energetically similar to the species (reactant,
intermediate or product) nearest to it on the reaction path. In
strongly exothermic reactions the transition state will resemble the
reactant species. In strongly endothermic conversions, such as that
shown to the right, the transition state will resemble the high-energy
intermediate or product, and will track the energy of this intermediate
if it changes. This change in transition state energy and activation
energy as the stability of the intermediate changes may be observed by
clicking the higher or lower buttons to the right of the energy diagram.
Three examples may be examined, and the reference curve is changed to
gray in the diagrams for higher (magenta) and lower (green) energy
intermediates.
The carbocation intermediate formed in the first step of the
addition reaction now assumes a key role, in that it directly influences
the activation energy for this step. Independent research shows that the
stability of carbocations varies with the nature of substituents, in a
manner similar to that seen for alkyl radicals. The exceptional
stability of allyl and benzyl cations is the result of charge
delocalization, and the stabilizing influence of alkyl substituents,
although less pronounced, has been interpreted in a similar fashion.
| Carbocation
Stability |
CH3(+) |
< |
CH3CH2(+) |
< |
(CH3)2CH(+) |
≈ |
CH2=CH-CH2(+) |
< |
C6H5CH2(+) |
≈ |
(CH3)3C(+) |
|
From this information, applying the Hammond Postulate, we arrive at
a plausible rationaliization of Markovnikov's rule. When an
unsymmetrically substituted double bond is protonated, we expect the
more stable carbocation intermediate to be formed faster than the less
stable alternative, because the activation energy of the path to the
former is the lower of the two possibilities. This is illustrated by the
following equation for the addition of hydrogen chloride to propene.
Note that the initial acid-base equilibrium leads to a pi-complex which
immediately reorganizes to a sigma-bonded carbocation intermediate. The
more stable 2º-carbocation is formed preferentially, and the conjugate
base of the Brønsted acid (chloride anion in the example shown below)
then rapidly bonds to this electrophilic intermediate to form the final
product.

The following energy diagram summarizes these features. Note that
the pi-complex is not shown, since this rapidly and reversibly formed
species is common to both possible reaction paths.
The formation of carbocations is sometimes accompanied by a
structural rearrangement. Such rearrangements take place by a shift of a
neighboring alkyl group or hydrogen, and are favored when the rearranged
carbocation is more stable than the initial cation. The addition of HCl
to 3,3-dimethyl-1-butene, for example, leads to an unexpected product,
2-chloro-2,3-dimethylbutane, in somewhat greater yield than
3-chloro-2,2-dimethylbutane, the expected Markovnikov product. This
supprising result may be explained by a carbocation rearrangement of the
initially formed 2º-carbocation to a 3º-carbocation by a 1,2-shift of a
methyl group. To see this rearrangement click the "Show Mechanism"
button to the right of the equation.
Another factor that may induce rearrangement of carbocation
intermediates is strain. The addition of HCl to α-pinene, the major
hydrocarbon component of turpentine, gives the rearranged product,
bornyl chloride, in high yield. As shown in the following equation, this
rearrangement converts a 3º-carbocation to a 2º-carbocation, a
transformation that is normally unfavorable. However, the rearrangement
also expands a strained four-membered ring to a much less-strained
five-membered ring, and this relief of strain provides a driving force
for the rearrangement. A three-dimensional projection view of the
rearrangement may be seen by clicking the "Other View" button.
The atom numbers (colored red) for the pinene structure are retained
throughout the rearrangement to help orient the viewer. The green
numbers in the final product represent the proper numbering of this
bicyclic ring system.
The propensity for structural rearrangement shown by certain molecular
constitutions, as illustrated above.
3. Addition of Lewis Acids
(Electrophilic Reagents)
The proton is not the only electrophilic species that initiates
addition reactions to the double bond. Lewis acids like the halogens,
boron hydrides and certain transition metal ions are able to bond to the
alkene pi-electrons, and the resulting complexes rearrange or are
attacked by nucleophiles to give addition products. The electrophilic
character of the halogens is well known. Although fluorine is
uncontrollably reactive, chlorine, bromine and to a lesser degree iodine
react selectively with the double bond of alkenes. The addition of
chlorine and bromine to alkenes, as shown in the following general
equation, proceeds by an initial electrophilic attack on the
pi-electrons of the double bond. Iodine adds reversibly to double bonds,
but the equilibrium does not normally favor the addition product, so it
is not a useful preparative method. Dihalo-compounds in which the
halogens are juxtaposed in the manner shown are called vicinal,
from the Latin vicinalis, meaning neighboring.
R2C=CR2
+ X2
——>
R2CX-CR2X
Other halogen containing reagents which add to double bonds include
hypohalous acids, HOX, and sulfenyl chlorides, RSCl. These
reagents are unsymmetrical, so their addition to unsymmetrical double
bonds may in principle take place in two ways. In practice, these
addition reactions are regioselective, with one of the two possible
constitutionally isomeric products being favored. The electrophilic
moiety of these reagents is the halogen.
(CH3)2C=CH2
+ HOBr
——>
(CH3)2COH-CH2Br
(CH3)2C=CH2
+ C6H5SCl
——>
(CH3)2CCl-CH2SC6H5
The regioselectivity of the above reactions may be explained by the
same mechanism we used to rationalize the Markovnikov rule. Thus,
bonding of an electrophilic species to the double bond of an alkene
should result in preferential formation of the more stable (more highly
substituted) carbocation, and this intermediate should then combine
rapidly with a nucleophilic species to produce the addition product.
This is illustrated by the following equation.
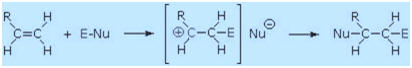
To apply this mechanism we need to determine the electrophilic moiety in each
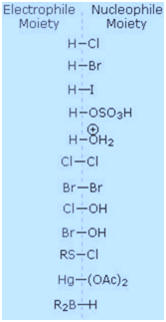
of the reagents. By using electronegativity differences we can dissect
common addition reagents into electrophilic and nucleophilic moieties,
as shown on the right. In the case of hypochlorous and hypobromous acids (HOX), these weak Brønsted acids (pKa's
ca. 8) do not react as proton donors; and since oxygen is more
electronegative than chlorine or bromine, the electrophile will be a
halide cation. The nucleophilic species that bonds to the intermediate
carbocation is then hydroxide ion, or more likely water (the usual
solvent for these reagents), and the products are called halohydrins.
Sulfenyl chlorides add in the opposite manner because the electrophile
is a sulfur cation, RS(+),
whereas the nucleophilic moiety is chloride anion (chlorine is more
electronegative than sulfur
The addition products formed in reactions of alkenes with mercuric acetate and boron
hydrides (compounds shown at the bottom of of the reagent list) are
normally not isolated, but instead are converted to alcohols by a
substitution reaction. These important synthetic transformations are
illustrated for 2-methylpropene by the following equations, in which the
electrophilic moiety is colored red and the nucleophile blue. The top
reaction sequence illustrates the
oxymercuration procedure and the bottom is an example of
hydroboration.
The light blue vertical line separates the addition reaction on the left from
the substitution on the right. The atoms or groups that have been added
to the original double bond are colored orange in the final product. In
both cases the overall reaction is the addition of water to the double
bond, but the regioselectivity is reversed. The
oxymercuration reaction gives the product predicted by Markovnikov's
rule; hydroboration on the other hand gives the "anti-Markovnikov"
product. Complementary reactions such as these are important because
they allow us to direct a molecular transformation whichever way is
desired.
Mercury and boron are removed from the organic substrate in the second
step of oxymercuration and hydroboration respectively. These reactions
are seldom discussed in detail; however, it is worth noting that the
mercury moiety is reduced to metallic mercury by borohydride (probably
by way of radical intermediates), and boron is oxidized to borate by the
alkaline peroxide. Addition of hydroperoxide anion to the electrophilic
borane generates a tetra-coordinate boron peroxide, having the general
formula R3B-O-OH(-).
This undergoes successive intramolecular shifts of alkyl groups from
boron to oxygen, accompanied in each event by additional peroxide
addition to electron deficient boron. The retention of configuration of
the migrating alkyl group is attributed to the intramolecular nature of
the rearrangement.
Since the oxymercuration sequence gives the same hydration product as
acid-catalyzed addition of water
(see Brønsted acid addition), we might question why this
two-step procedure is used at all. The reason lies in the milder
reaction conditions used for oxymercuration. The strong acid used for
direct hydration may not be tolerated by other functional groups, and in
some cases may cause molecular rearrangement
(see above).
The addition of borane, BH3,
requires additional comment. In pure form this reagent is a dimeric gas
B2H6,
called diborane, but in ether or THF solution it is dissociated into a
solvent coordinated monomer, R2OBH3.
Diborane itself does not react easily with alkene double bonds; however,
the solvated monomer adds rapidly under mild conditions. Boron and
hydrogen have rather similar electronegativities, with hydrogen being
slightly greater, so it is not likely there is significant dipolar
character to the B-H bond. Since boron is electron deficient (it does
not have a valence shell electron octet) the reagent itself is a Lewis
acid and can bond to the pi-electrons of a double bond by displacement
of the ether moiety from the solvated monomer. As shown in the following
equation, this bonding might generate a dipolar intermediate consisting
of a negatively-charged boron and a carbocation. Such a species would
not be stable and would rearrange to a neutral product by the shift of a
hydride to the carbocation center. Indeed, this hydride shift is
believed to occur concurrently with the initial bonding to boron, as
shown by the transition state drawn below the equation, so the discrete
intermediate shown in the equation is not actually formed. Nevertheless,
the carbocation stability rule cited above remains a useful way to
predict the products from hydroboration reactions. You may correct
the top equation by clicking the button on its right. Note that this
addition is unique among those we have discussed, in that it is a
single-step process. Also, all three hydrogens in borane are potentially
reactive, so that the alkyl borane product from the first addition may
serve as the hydroboration reagent for two additional alkene molecules.
Stereoselectivity in Addition
Reactions to Double Bonds
As illustrated in the drawing on the right, the pi-bond fixes the
carbon-carbon double bond in a planar configuration, and does not permit
free rotation about the double bond itself. We see then that addition
reactions to this function might occur in three different ways,
depending on the relative orientation of the atoms or groups that add to
the carbons of the double bond: (i) they may bond from the same side,
(ii) they may bond from opposite sides, or (iii) they may bond randomly
from both sides. The first two possibilities are examples of
stereoselectivity, the first being termed syn-addition,
and the second anti-addition. Since initial electrophilic attack
on the double bond may occur equally well from either side, it is in the
second step (or stage) of the reaction (bonding of the nucleophile) that
stereoselectivity may be imposed.
If the two-step mechanism described above is correct, and if the
carbocation intermediate is sufficiently long-lived to freely-rotate
about the sigma-bond component of the original double bond, we would
expect to find random or non-stereoselective addition in the products.
On the other hand, if the intermediate is short-lived and factors such
as steric hindrance or neighboring group interactions favor one side in
the second step, then stereoselectivity in product formation is likely.
The following table summarizes the results obtained from many studies,
the formula HX refers to all the strong Brønsted acids. The interesting
differences in stereoselectivity noted here provide further insight into
the mechanisms of these addition reactions.
|
Reagent |
H–X |
X2 |
HO–X |
RS–Cl |
Hg(OAc)2 |
BH3 |
|
Stereoselectivity |
mixed |
anti |
anti |
anti |
anti |
syn |
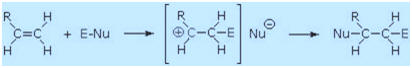
1. Brønsted Acid Additions
The stereoselectivity of Brønsted acid addition is sensitive to
experimental conditions such as temperature, solvent and reagent
concentration. The selectivity is often anti, but reports of syn
selectivity and non-selectivity are not uncommon. Of all the reagents
discussed here, these strong acid additions (E = H in the following
equation) come closest to proceeding by the proposed two-step mechanism
in which a discrete carbocation intermediate is generated in the first
step. Such reactions are most prone to rearrangement when this is
favored by the alkene structure.
2. Addition Reactions Initiated
by Electrophilic Halogen
The halogens chlorine and bromine add rapidly to a wide variety of
alkenes without inducing the kinds of structural rearrangements noted
for strong acids (first example below). The stereoselectivity of these
additions is strongly anti, as shown in many of the following
examples.

An important principle should be restated at this time. The alkenes
shown here are all achiral, but the addition products have chiral
centers, and in many cases may exist as enantiomeric stereoisomers. In
the absence of chiral catalysts or reagents, reactions of this kind will
always give racemic mixtures if the products are enantiomeric. On the
other hand, if two chiral centers are formed in the addition the
reaction will be diastereomer selective. This is clearly shown by the
addition of bromine to the isomeric 2-butenes. Anti-addition to
cis-2-butene gives the racemic product, whereas anti-addition to the
trans-isomer gives the meso-diastereomer.
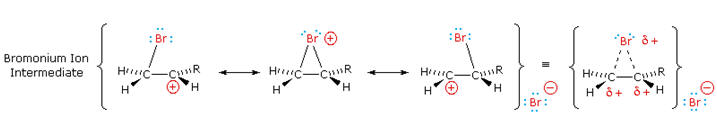
We can account both for the high stereoselectivity and the lack of
rearrangement in these reactions by proposing a stabilizing interaction
between the developing carbocation center and the electron rich halogen
atom on the adjacent carbon. This interaction, which is depicted for
bromine in the following equation, delocalizes the positive charge on
the intermediate and blocks halide ion attack from the syn-location.

The stabilization provided by this halogen-carbocation bonding
makes rearrangement unlikely. In a few cases three-membered cyclic
halonium cations have been isolated and identified as true
intermediates. A resonance description of such a bromonium ion
intermediate is shown below. The positive charge is delocalized over all
the atoms of the ring, but should be concentrated at the more
substituted carbon (carbocation stability), and this is the site to
which the nucleophile will bond.
Because they proceed by way of polar ion-pair intermediates, chlorine
and bromine addition reactions are faster in polar solvents than in
non-polar solvents, such as hexane or carbon tetrachloride. However, in
order to prevent solvent nucleophiles from competing with the halide
anion, these non-polar solvents are often selected for these reactions.
In water or alcohol solution the nucleophilic solvent may open the
bromonium ion intermediate to give an α-halo-alcohol or ether, together
with the expected vic-dihalide. Such reactions are sensitive to pH and
other factors, so when these products are desired it is necessary to
modify the addition reagent. Aqueous chlorine exists as the following
equilibrium, Keq
≈ 10-4.
By adding AgOH, the concentration of HOCl can be greatly increased, and
the chlorohydrin addition product obtained from alkenes.
|
Cl2 + H2O |
 |
HOCl + HCl |
The more widely used HOBr reagent, hypobromous acid, is commonly
made by hydrolysis of N-bromoacetamide, as shown below. Both HOCl and
HOBr additions occur in an anti fashion, and with the regioselectivity
predicted by this mechanism (OH bonds to the more substituted carbon of
the alkene).
|
CH3CONHBr + H2O |
 |
HOBr + CH3CONH2 |
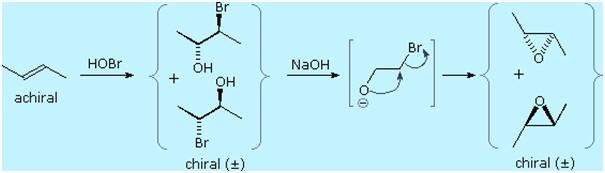
Vicinal halohydrins provide an alternative route for the
epoxidation of alkenes over that of
reaction with peracids. As illustrated in the following
diagram, a base induced intramolecular
substitution reaction forms a three-membered cyclic ether
called an epoxide. Both the halohydrin formation and halide displacement
reactions are stereospecific, so stereoisomerism in the alkene will be
reflected in the epoxide product (i.e. trans-2-butene forms a
trans-disubstituted epoxide). A general procedure for forming these
useful compounds will be discussed in the next section.
3. Addition Reactions Involving Other Cyclic Onium Intermediates
Sulfenyl
chloride additions are initiated by the attack of an electrophilic
sulfur species on the pi-electrons of the double bond. The resulting
cationic intermediate may be stabilized by the non-bonding valence shell
electrons on the sulfur in exactly the same way the halogens exerted
their influence. Indeed, a cyclic sulfonium ion intermediate analogous
to the bromonium ion is believed to best represent this intermediate
.
Two advantages of the oxymercuration method of adding water to a double
bond are its high anti-stereoselectivity and the lack of rearrangement
in sensitive cases. These characteristics are attributed to a
mercurinium ion intermediate, analogous to the bromonium ion discussed
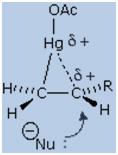
above. In this case it must be d-orbital electrons that are involved in
bonding to carbon. A drawing of this intermediate is shown on the right.
Hydroboration
Stereoselectivity
The hydroboration reaction is among the few simple addition reactions
that proceed cleanly in a syn fashion.
As noted
above,
this is a single-step reaction. Since the bonding of the double bond
carbons to boron and hydrogen is concerted, it follows that the
geometry of this addition must be syn. Furthermore, rearrangements
are unlikely inasmuch as a discrete carbocation intermediate is never
formed. These features are illustrated for the hydroboration of α-pinene
in the following equation. Since the hydroboration procedure is most
commonly used to hydrate alkenes in an anti-Markovnikov fashion, we also
need to know the stereoselectivity of the second oxidation reaction,
which substitutes a hydroxyl group for the boron atom. Independent study
has shown this reaction takes place with retention of configuration
so the overall addition of water is also syn.
The
hydroboration of α-pinene also provides a nice example of steric
hindrance control in a chemical reaction. In the less complex alkenes
used in earlier examples the plane of the double bond was often a plane
of symmetry, and addition reagents could approach with equal ease from
either side. In this case, one of the methyl groups bonded to C-6 (colored
blue in the equation) covers one face of the double bond, blocking any
approach from that side. All reagents that add to this double bond must
therefore approach from the side opposite this methyl.
4. Hydrogenation
Addition of
hydrogen to a carbon-carbon double bond is called hydrogenation.
The overall effect of such an addition is the reductive removal of the
double bond functional group. Regioselectivity is not an issue, since
the same group (a hydrogen atom) is bonded to each of the double bond
carbons. The simplest source of two hydrogen atoms is molecular hydrogen
(H2),
but mixing alkenes with hydrogen does not result in any discernable
reaction. Although the overall hydrogenation reaction is exothermic, a
high activation energy prevents it from taking place under normal
conditions. This restriction may be circumvented by the use of a
catalyst, as shown in the following diagram.
Catalysts are
substances that changes the rate (velocity) of a chemical reaction
without being consumed or appearing as part of the product. Catalysts
act by lowering the activation energy of reactions, but they do not
change the relative potential energy of the reactants and products.
Finely divided metals, such as platinum, palladium and nickel, are among
the most widely used hydrogenation catalysts. Catalytic hydrogenation
takes place in at least two stages, as depicted in the diagram. First,
the alkene must be adsorbed on the surface of the catalyst along with
some of the hydrogen. Next, two hydrogens shift from the metal surface
to the carbons of the double bond, and the resulting saturated
hydrocarbon, which is more weakly adsorbed, leaves the catalyst surface.
The exact nature and timing of the last events is not well understood.
As shown in the energy diagram, the hydrogenation of alkenes is
exothermic, and heat is released corresponding to the ΔE (colored green)
in the diagram. This heat of reaction can be used to evaluate the
thermodynamic stability of alkenes having different numbers of alkyl
substituents on the double bond. For example, the following table lists
the heats of hydrogenation for three C5H10
alkenes which give the same alkane product (2-methylbutane). Since a
large heat of reaction indicates a high energy reactant, these heats are
inversely proportional to the stabilities of the alkene isomers. To a
rough approximation, we see that each alkyl substituent on a double bond
stabilizes this functional group by a bit more than 1 kcal/mole.
|
Alkene Isomer |
(CH3)
2CHCH=CH2
3-methyl-1-butene |
CH2=C(CH3)CH2CH3
2-methyl-1-butene |
(CH3)2C=CHCH3
2-methyl-2-butene |
|
Heat of Reaction
( ΔHº ) |
–30.3 kcal/mole |
–28.5 kcal/mole |
–26.9 kcal/mole |
|
|
|
From
the mechanism shown here we would expect the addition of
hydrogen to occur with syn-stereoselectivity. This is often
true, but the hydrogenation catalysts may also cause
isomerization of the double bond prior to hydrogen addition, in
which case stereoselectivity may be uncertain.
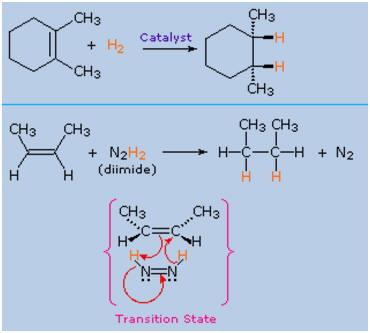
A non-catalytic procedure for the syn-addition of hydrogen makes
use of the unstable compound diimide, N2H2.
This reagent must be freshly generated in the reaction system,
usually by oxidation of hydrazine, and the strongly exothermic
reaction is favored by the elimination of nitrogen gas (a very
stable compound). Diimide may exist as cis-trans isomers; only
the cis-isomer serves as a reducing agent. Examples of alkene
reductions by both procedures are shown below.
|
5. Oxidations
(i) Hydroxylation
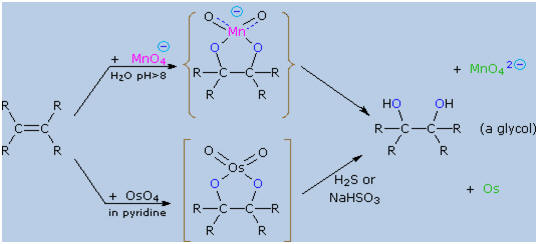
Dihydroxylated
products (glycols) are obtained by reaction with aqueous potassium
permanganate (pH > 8) or osmium tetroxide in pyridine solution. Both
reactions appear to proceed by the same mechanism (shown below); the
metallocyclic intermediate may be isolated in the osmium reaction. In
basic solution the purple permanganate anion is reduced to the green
manganate ion, providing a nice color test for the double bond
functional group. From the mechanism shown here we would expect
syn-stereoselectivity in the bonding to oxygen, and regioselectivity is
not an issue.
When viewed in context with the previously discussed addition reactions,
the hydroxylation reaction might seem implausible. Permanganate and
osmium tetroxide have similar configurations, in which the metal atom
occupies the center of a tetrahedral grouping of negatively charged
oxygen atoms. How, then, would such a species interact with the
nucleophilic pi-electrons of a double bond? A possible explanation is
that an empty d-orbital of the electrophilic metal atom extends well
beyond the surrounding oxygen atoms and initiates electron transfer from
the double bond to the metal. Back-bonding of the nucleophilic oxygens
to the antibonding pi orbital completes this interaction. The result is
formation of a metallocyclic intermediate, as shown.
(ii) Epoxidation
Some oxidation
reactions of alkenes give cyclic ethers in which both carbons of a
double bond become bonded to the same oxygen atom. These products are
called epoxides or oxiranes. An important method for
preparing epoxides is by reaction with peracids, RCO3H.
The oxygen-oxygen bond of such peroxide derivatives is not only weak
(ca. 35 kcal/mole), but in this case is polarized so that the acyloxy
group is negative and the hydroxyl group is positive (recall that the
acidity of water is about ten powers of ten weaker than that of a
carboxylic acid). If we assume electrophilic character for the OH
moiety, the following equation may be written.
It is unlikely
that a dipolar intermediate, as shown above, is actually formed. The
epoxidation reaction is believed to occur in a single step with a
transition state incorporating all of the bonding events shown in the
equation. Consequently, epoxidations by peracids always have
syn-stereoselectivity, and seldom give structural rearrangement. You may
see the transition state by clicking the
Change
Equation button.
Presumably the electron shifts indicated by the blue arrows induce a
charge separation that is immediately neutralized by the green arrow
electron shifts.
The previous
few reactions have been classified as reductions or oxidations,
depending on the
change
in oxidation state of the functional carbons.
It is important to remember that whenever an atom or group is reduced,
some other atom or group is oxidized, and a balanced equation must
balance the electron gain in the reduced species with the electron loss
in the oxidized moiety, as well as numbers and kinds of atoms. Starting
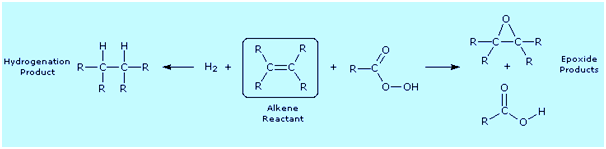
from an alkene (drawn in the box), the following diagram shows a
hydrogenation reaction on the left (the catalyst is not shown) and an
epoxidation reaction on the right. Examine these reactions, and for each
identify which atoms are reduced and which are oxidized.
Epoxides may
be cleaved by aqueous acid to give glycols that are often diastereomeric
with those prepared by the
syn-hydroxylation
reaction
described above. Proton transfer from the acid catalyst generates the
conjugate acid of the epoxide, which is attacked by nucleophiles such as
water in the same way that the cyclic bromonium ion described above
undergoes reaction. The result is anti-hydroxylation of the
double bond, in contrast to the syn-stereoselectivity of the earlier
method. In the following equation this procedure is illustrated for a
cis-disubstituted epoxide, which, of course, could be prepared from the
corresponding cis-alkene. This hydration of an epoxide does not change
the oxidation state of any atoms or groups.

(iii) Oxidative Cleavage of Double Bonds
Ozonolysis
In determining the structural formula of an alkene, it is often
necessary to find the location of the double bond within a given carbon
framework. One way of accomplishing this would be to selectively break
the double bond and mark the carbon atoms that originally formed that
bond. For example, there are three isomeric alkenes that all give
2-methylbutane on catalytic hydrogenation. These are 2-methyl-2-butene
(compound A), 3-methyl-1-butene (compound B) and 2-methyl-1-butene
(compound C), shown in the following diagram. If the double bond is
cleaved and the fragments marked at the cleavage sites, the location of
the double bond is clearly determined for each case. A reaction that
accomplishes this useful transformation is known. It is called
ozonolysis, and its application to each of these examples may be
seen by clicking the "Show Reaction" button.
Ozone, O3,
is an allotrope of oxygen that adds rapidly to carbon-carbon double
bonds. Since the overall change in ozonolysis is more complex than a
simple addition reaction, its mechanism has been extensively studied.
Reactive intermediates called ozonides have been isolated from the
interaction of ozone with alkenes, and these unstable compounds may be
converted to stable products by either a reductive workup (Zn dust in
water or alcohol) or an oxidative workup (hydrogen peroxide). The
results of an oxidative workup may be seen by clicking the "Show
Reaction" button a second time. Continued clicking of this button
repeats the cycle. The chief difference in these conditions is that
reductive workup gives an aldehyde product when hydrogen is present on a
double bond carbon atom, whereas oxidative workup gives a carboxylic
acid or carbon dioxide in such cases. The following equations illustrate
ozonide formation, a process that is believed to involve initial syn-addition
of ozone, followed by rearrangement of the extremely unstable molozonide
addition product. They also show the decomposition of the final ozonide
to carbonyl products by either a reductive or oxidative workup.
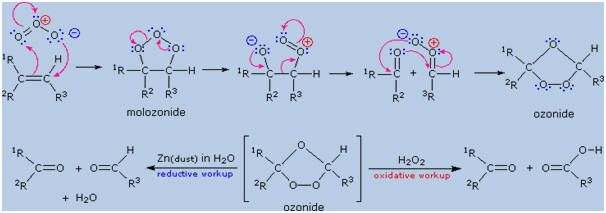
|
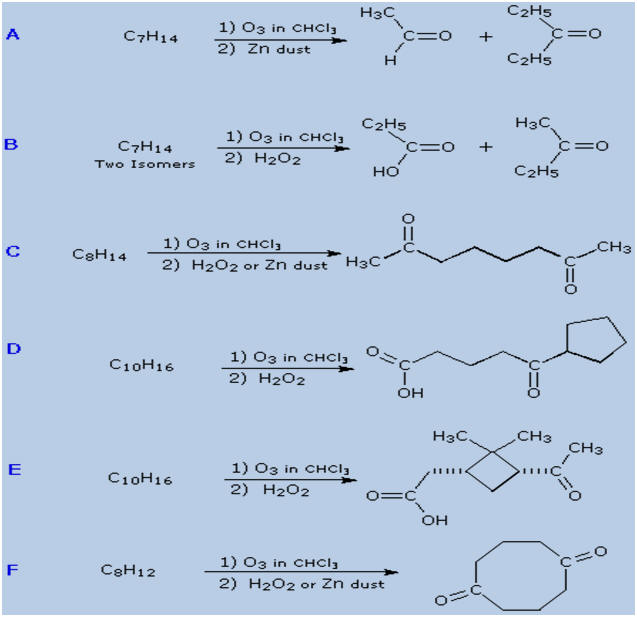
From this analysis and the examples given here, you
should be able to deduce structural formulas for the
alkenes that give the following ozonolysis products. |
|
|
|
|
|
Glycol
Cleavage
The vicinal glycols prepared by alkene hydroxylation (reaction with
osmium tetroxide or permanganate) are cleaved to aldehydes and ketones
in high yield by the action of lead tetraacetate (Pb(OAc)4)
or periodic acid (HIO4).
This oxidative cleavage of a carbon-carbon single bond provides a
two-step, high-yield alternative to ozonolysis, that is often preferred
for small scale work involving precious compounds. A general equation
for these oxidations is shown below. As a rule, cis-glycols react more
rapidly than trans-glycols, and there is evidence for the intermediacy
of heterocyclic intermediates (as shown), although their formation is
not necessary for reaction to occur.
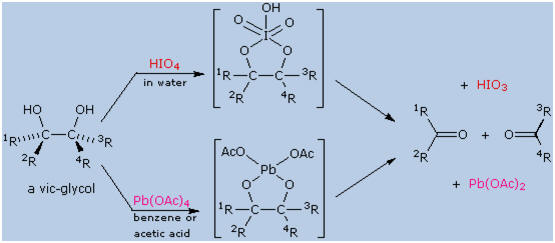
From this analysis and the examples given here, you
should be able to deduce structural formulas for the
alkenes that give the following ozonolysis products
Free Radical Reactions of Alkenes
1. Addition of Radicals to Alkenes
Protons and
other electrophiles are not the only reactive species that initiate
addition reactions to carbon-carbon double bonds. Curiously, this first
became evident as a result of conflicting reports concerning the
regioselectivity of HBr additions. As noted earlier, the acid-induced
addition of HBr to 1-butene gave predominantly 2-bromobutane, the
Markovnikov Rule
product. However, in some early experiments in which peroxide
contaminated reactants were used, 1-bromobutane was the chief product.
Further study showed that an alternative radical chain-reaction,
initiated by peroxides, was responsible for the anti-Markovnikov
product. This is shown by the following equations.
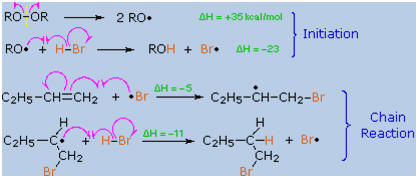
The weak O–O
bond of a peroxide initiator is broken homolytically by thermal or hight
energy. The resulting alkoxy radical then abstracts a hydrogen atom from
HBr in a strongly exothermic reaction. Once a bromine atom is formed it
adds to the π-bond of the alkene in the first step of a chain reaction.
This addition is regioselective, giving the more stable
carbon
radical
as an intermediate. The second step is carbon radical abstraction of
another hydrogen from HBr, generating the anti-Markovnikov alkyl bromide
and a new bromine atom. Each of the steps in this chain reaction is
exothermic, so once started the process continues until radicals are
lost to termination events.
This free radical chain addition competes very favorably with the slower
ionic addition of HBr described earlier, especially in non-polar
solvents. It is important to note, however, that HBr is unique in this
respect. The radical addition process is unfavorable for HCl and HI
because one of the chain steps becomes endothermic (the second for HCl &
the first for HI).
Other radical addition reactions to alkenes have been observed, one
example being the peroxide induced addition of carbon tetrachloride
shown in the following equation
|
RCH=CH2
+
CCl4 (peroxide
initiator)
—>
RCHClCH2CCl3 |
The best known
and most important use of free radical addition to alkenes is probably
polymerization.
Since the addition of carbon radicals to double bonds is energetically
favorable, concentrated solutions of alkenes are prone to
radical-initiated polymerization, as illustrated for propene by the
following equation. The blue colored R-group represents an initiating
radical species or a growing polymer chain; the propene monomers are
colored maroon. The addition always occurs so that the more stable
radical intermediate is formed.
|
RCH2(CH3)CH·
+
CH3CH=CH2
—>
RCH2(CH3)CH-CH2(CH3)CH·
+
CH3CH=CH2
—>
RCH2(CH3)CHCH2(CH3)CH-CH2(CH3)CH·
—>
etc. |
2. Allylic Substitution
We noted
earlier that benzylic and allylic sites are exceptionally reactive in
free
radical halogenation reactions.
Since carbon-carbon double bonds add chlorine and bromine in liquid
phase solutions, radical substitution reactions by these halogens are
often carried out at elevated tempreature in the gas phase (first
equation below). Formation of the ionic π-complexes that are
intermediates in halogen addition is unfavorable in the absence of polar
solvents, and
entropy
generally favors substitution over addition.
The brominating reagent, N-bromosuccinimide (NBS), has proven useful for
achieving allylic or benzylic substitution in CCl4
at temperatures below its boiling point (77 0C). One such application is
shown in the second equation.
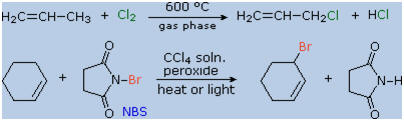
The
predominance of allylic substitution over addition in the NBS reaction
is interesting. The N–Br bond is undoubtedly weak (probably less than 50
kcal/mol) so bromine atom abstraction by radicals should be very
favorable. The resulting succinimyl radical might then establish a chain
reaction by removing an allylic hydrogen from the alkene. One problem
with this mechanism is that NBS is very insoluble in CCl4,
about 0.006 mole / liter at reflux. Although it is possible that the
allylic bromination occurs at a solid-liquid interface, evidence for
another pathway has been obtained. In the non-polar solvent used for
these reactions, very low concentrations of bromine may be generated
from NBS. This would serve as a source of bromine atoms, which would
abstract allylic hydrogens irreversibly (an exothermic reaction) in
competition with reversible addition to the double bond. The HBr
produced in this way is known to react with NBS, giving a new bromine
molecule and succinimide, as shown here. Ionic addition of bromine to
the double bond would be very slow in these circumstances.
|
HBr + (CH2CO)2NBr
—>
Br2
+ (CH2CO)2NH |
This mechanism
is essentially the same as that for the
free
radical halogenation
of alkanes, with NBS serving as a source of very low concentrations of
bromine. Unsymmetrical allylic radicals will react to give two
regioisomers. Thus, 1-octene on bromination with NBS yields a mixture of
3-bromo-1-octene (ca. 18%) and 1-bromo-2-octene (82%) - both cis and
trans isomers.
|
RCH2CH=CH2
+ (CH2CO)2NBr
—>
RCHBrCH=CH2
+ RCH=CHCH2Br
+ (CH2CO)2NH |
Dienes
1. Properties of Dienes
When
considering compounds having two or more double bonds in a molecule, it
is useful to identify three distinct ways in which these functions may
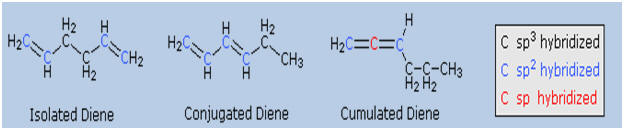
be oriented with respect to each other. First, the double bonds may be
separated by one or more sp3-hybridized
carbon atoms, as in 1,5-hexadiene. In this circumstance each double bond
behaves independently of the other, and we refer to them as isolated.
A second relationship has the double bonds connected to each other by a
single bond, as in 1,3-hexadiene, and we refer to this arrangement as
conjugated. Finally, two double bonds might share a carbon atom, as
in 1,2-hexadiene. The central carbon atom in such a system is
sp-hybridized, and we call such double bonds cumulated. These
three isomers are shown in the following diagram, and three other
similar isomers will be displayed on clicking the Change Examples
button. In cases where stereoisomers are possible only the E-isomer is
shown.
Another stereoisomeric factor associated with conjugated dienes will be
demonstrated by clicking the Change Examples button a second
time. Rotation about the single bond joining the two double bonds (colored
blue) converts a trans-like s-trans conformation to its s-cis
form. The energy barrier to this conformational isomerisation is
normally low, and the s-trans conformer is often more stable than the s-cis
conformer, as shown in the diagram.
These
categories are based on more than obvious structural variations. We find
significant differences in the chemical properties of dienes depending
on their structural type. For
example, catalytic hydrogenation converts all the dienes shown here to
the alkane hexane, but the heats of reaction (heat
of hydrogenation)
reflect characteristic differences in their thermodynamic stability.
This is illustrated in the diagram on the right. Taking the heat of
hydrogenation of 1-hexene (30.1 kcal/mole) as a reference, we find that
the isolated diene, 1,5-hexadiene, as expected, generates double this
heat of reaction on conversion to hexane. The cumulated diene,
1,2-hexadiene, has a 6 kcal/mole higher heat of reaction, indicating it
is less stable than the isolated diene by this magnitude. On the other
hand, conjugation of double bonds seems to stabilize a diene by about 5
kcal/mole. The increase in stability of 2,4-hexadiene over 1,3-hexadiene
(both are conjugated) is due to the increased double bond substitution
of the former, a factor noted
earlier
for simple alkenes.
The stabilization of dienes by conjugation is less dramatic than the
aromatic stabilization
of benzene.
Nevertheless, similar resonance and molecular orbital descriptions of
conjugation may be written. A resonance description, such as the one
shown here, involves charge separation, implying a relatively small
degree of stabilization.
|
CH2=CH-CH=CH2
(+)CH2-CH=CH-CH2:(–) |
A molecular
orbital model for 1,3-butadiene is shown below. Note that the lobes of
the four p-orbital components in each pi-orbital are colored differently
and carry a plus or minus sign. This distinction refers to different
phases, defined by the mathematical wave equations for such orbitals.
Regions in which adjacent orbital lobes undergo a phase change are
called nodes. Orbital electron density is zero in such regions.
Thus a single p-orbital has a node at the nucleus, and all the
pi-orbitals shown here have a nodal plane that is defined by the atoms
of the diene. This is the only nodal surface in the lowest energy
pi-orbital, π1.
Higher energy pi-orbitals have an increasing number of nodes.
2. Addition Reactions of Dienes
Addition
reactions of isolated dienes proceed more or less as expected from the
behavior of simple alkenes. Thus, if one molar equivalent of
1,5-hexadiene is treated with one equivalent of bromine a mixture of
5,6-dibromo-1-hexene, 1,2,5,6-tetrabromohexane and unreacted diene is
obtained, with the dibromo compound being the major product (about 50%).
|
CH2=CH(CH2)2CH=CH2
+ Br2
|
BrCH2CHBr(CH2)2CH=CH2
+ |
BrCH2CHBr(CH2)2CHBrCH2Br
+ |
CH2=CH(CH2)2CH=CH2 |
| |
5,6-dibromo-1-hexene |
1,2,5,6-tetrabromohexane |
1,5-hexadiene |
Similar
reactions of conjugated dienes, on the other hand, often give unexpected
products. The addition of bromine to 1,3-butadiene is an example. As
shown below, a roughly 50:50 mixture of 3,4-dibromo-1-butene (the
expected product) and 1,4-dibromo-2-butene (chiefly the E-isomer) is
obtained. The latter compound is remarkable in that the remaining double
bond is found in a location where there was no double bond in the
reactant. This interesting relocation requires an explanation.
|
CH2=CH-CH=CH2
+ Br2
|
BrCH2CHBr-CH=CH2
+ |
BrCH2CH=CHCH2Br |
| |
3,4-dibromo-1-butene |
1,4-dibromo-2-butene |
The expected
addition product from reactions of this kind is the result of
1,2-addition, i.e. bonding to the adjacent carbons of a double bond.
The unexpected product comes from 1,4-addition, i.e. bonding at
the terminal carbon atoms of a conjugated diene with a shift of the
remaining double bond to the 2,3-location. These numbers refer to the
four carbons of the conjugated diene and are not IUPAC nomenclature
numbers. Product compositions are often temperature dependent, as the
addition of HBr to 1,3-butadiene demonstrates.
|
CH2=CH-CH=CH2
+ HBr
reaction temperature |
CH3CHBr-CH=CH2
+
1,2
addition yield |
CH3CH=CHCH2Br
1,4
addition yield |
|
0
ºC
40 ºC |
70%
15% |
30%
85% |
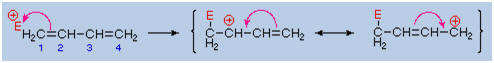
Bonding of an
electrophilic atom or group to one of the end carbon atoms of a
conjugated diene (#1) generates an allyl cation intermediate. Such
cations are stabilized by charge delocalization, and it is this
delocalization that accounts for the 1,4-addition product produced in
such addition reactions. As shown in the diagram, the positive charge is
distributed over carbons #2 and #4 so it is at these sites that the
nucleophilic component bonds. Note that resonance stabilization of the
allyl cation is greater than comparable
stabilization of 1,3-butadiene,
because charge is delocalized in the former, but created and separated
in the latter.
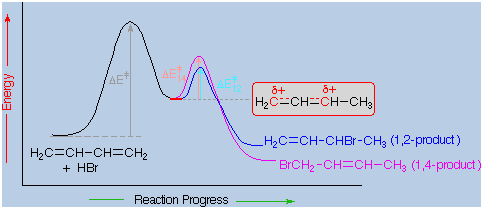
An explanation
for the temperature influence is shown in the following energy diagram
for the addition of HBr to 1,3-butadiene. The initial step in which a
proton bonds to carbon #1 is the rate determining step, as
indicated by the large activation energy (light gray arrow). The second
faster step is the product determining step, and there are two
reaction paths (colored blue for 1,2-addition and magenta for
1,4-addition). The 1,2-addition has a smaller activation energy than
1,4-addition, but the 1,4-product is more stable than the 1,2-product.
At low temperatures, the products are formed irreversibly and reflect
the relative rates of the two competing reactions. This is termed
kinetic control. At higher temperatures, equilibrium is established
between the products, and the thermodynamically favored
1,4-product dominates.
3. Diels-Alder Cycloaddition
The unique
character of conjugated dienes manifests itself dramatically in the
Diels-Alder
Cycloaddition Reaction.
A cycloaddition reaction is the concerted bonding together of two
independent pi-electron systems to form a new ring of atoms. When this
occurs, two pi-bonds are converted to two sigma-bonds, the simplest
example being the hypothetical combination of two ethene molecules to
give cyclobutane. This does not occur under normal conditions, but the
cycloaddition of 1,3-butadiene to cyanoethene (acrylonitrile) does, and
this is an example of the Diels-Alder reaction. The following diagram
illustrates two cycloadditions, and introduces several terms that are
useful in discussing reactions of this kind.
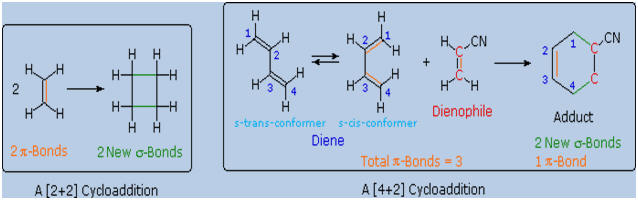
In the
hypothetical ethylene dimerization on the left, each reactant molecule
has a pi-bond (colored orange) occupied by two electrons. The
cycloaddition converts these pi-bonds into new sigma-bonds (colored
green), and this transformation.is then designated a [2+2] cycloaddition,
to enumerate the reactant pi-electrons that change their bonding
location.
The Diels-Alder reaction is an important and widely used method for
making six-membered rings, as shown on the right. The reactants used in
such reactions are a conjugated diene, simply referred to as the
diene, and a double or triple bond coreactant called the
dienophile, because it combines with (has an affinity for) the
diene. The Diels-Alder cycloaddition is classified as a [4+2] process
because the diene has four pi-electrons that shift position in the
reaction and the dienophile has two.
The Diels-Alder reaction is a single step process, so the diene
component must adopt a cis-like conformation in order for the end carbon
atoms (#1 & #4) to bond simultaneously to the dienophile. Such
conformations are called s-cis, the s referring to the
single bond connecting the two double bonds. The s-cis and s-trans
conformers of 1,3-butadiene are shown in the preceding diagram. For many
acyclic dienes the s-trans conformer is more stable than the s-cis
conformer (due to steric crowding of the end groups), but the two are
generally in rapid equilibrium, permitting the use of all but the most
hindered dienes as reactants in Diels-Alder reactions. In its usual
form, the diene component is electron rich, and the best dienophiles are
electron poor due to electron withdrawing substituents such as CN, C=O &
NO2.
The initial bonding interaction reflects this electron imbalance, with
the two new sigma-bonds being formed simultaneously, but not necessarily
at equal rates.
Stereospecificity
We noted
earlier that addition reactions of alkenes often exhibited
stereoselectivity,
in that the reagent elements in some cases added syn and in other cases
anti to the the plane of the double bond. Both reactants in the
Diels-Alder reaction may demonstrate stereoisomerism, and when they do
it is found that the relative configurations of the reactants are
preserved in the product (the adduct). The following drawing illustrates
this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. The
trans relationship of the cyano groups in the dienophile is preserved in
the six-membered ring of the adduct. Likewise, if the terminal carbons
of the diene bear substituents, their relative configuration will be
retained in the adduct. Using the earlier terminology, we could say that
bonding to both the diene and the dienophile is syn. An alternative
description, however, refers to the planar nature of both reactants and
terms the bonding in each case to be suprafacial (i.e. to or from
the same face of each plane). This
stereospecificity
also confirms the synchronous nature of the 1,4-bonding that takes
place.
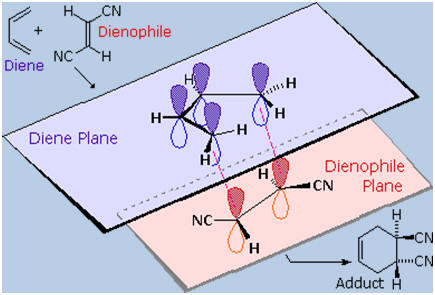
The
essential characteristics of the Diels-Alder cycloaddition reaction may
be summarized as follows:
(i)
The
reaction always creates a new six-membered ring. When intramolecular,
another ring may also be formed.
(ii) The diene component must be able to assume a s-cis
conformation.
(iii) Electron withdrawing groups on the dienophile facilitate
reaction.
(iv) Electron donating groups on the diene facilitate reaction.
(v) Steric hindrance at the bonding sites may inhibit or prevent
reaction.
(vi) The reaction is stereospecific with respect to substituent
configuration in both the dienophile and the diene.
There is no
reaction in example D because this diene cannot adopt a s-cis
orientation. In examples B, C, F, G & H at least one of the
reactants is cyclic so that the product has more than one ring, but the
newly formed ring is always six-membered. In example B the the
same cyclic compound acts as both the diene colored blue) and the
dienophile (colored red). The adduct has three rings, two of which are
the five-membered rings present in the reactant, and the third is the
new six-membered ring (shaded light yellow). Example C has an
alkyne as a dienophile (colored red). The initial Diels-Alder reaction
involves only one of the pi-bonds of the triple bond, so the adduct
retains a double bond at that location. This double bond could still
serve as a dienophile, but in the present case the diene is sufficiently
hindered to retard a second cycloaddition. The quinone dienophile in
reaction F has two dienophilic double bonds. However, the double
bond with two methyl substituents is less reactive than the
unsubstituted dienophile due in part to the electron donating properties
of the methyl groups and in part to steric hindrance. The
stereospecificity of the Diels-Alder reaction is demonstrated by
examples A, E & H. In A & H the stereogenic centers lie on
the dienophile, whereas in E these centers are on the diene. In
all cases the configuration of the reactant is preserved in the adduct.
Cyclic dienes,
such as those in examples B, C & G, give
bridged
bicyclic
adducts for which an additional configurational feature must be
designated. As shown in the following diagram, there are two possible
configurations for compounds of this kind. If a substituent (colored
orange here) is oriented cis to the longest or more unsaturated bridge (colored
blue here), it is said to be endo. When directed trans to the
bridge it is exo. When the Diels-Alder reaction forms bridged
bicyclic adducts and an unsaturated substituent is located on this
bicyclic structure (as in B & G), the chief product is normally
the endo isomer "Alder's Endo Rule". Example C does not
merit such a nomenclature, since stereoisomeric orientations of the
substituent are not possible.
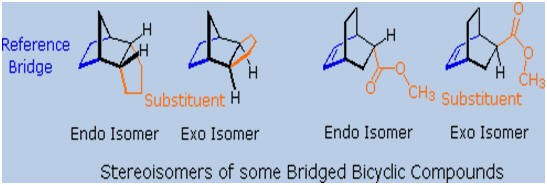
-
-
-
© M.EL-Fellah
,Chemistry Department, Garyounis University
|