|
|
![]()
Substituent Effects (contd.)
Here is a table that shows the effect of substituents
on a benzene ring have on both the rate and orientation of electrophilic aromatic
substitution reactions.
| Study Tip: This is a VERY important table ! It is worth knowing.... your best understanding will come if you learn HOW it works. It's application goes way beyond electrophilic aromatic substitution reactions. Key concepts to review ? Resonance and electronegativity |
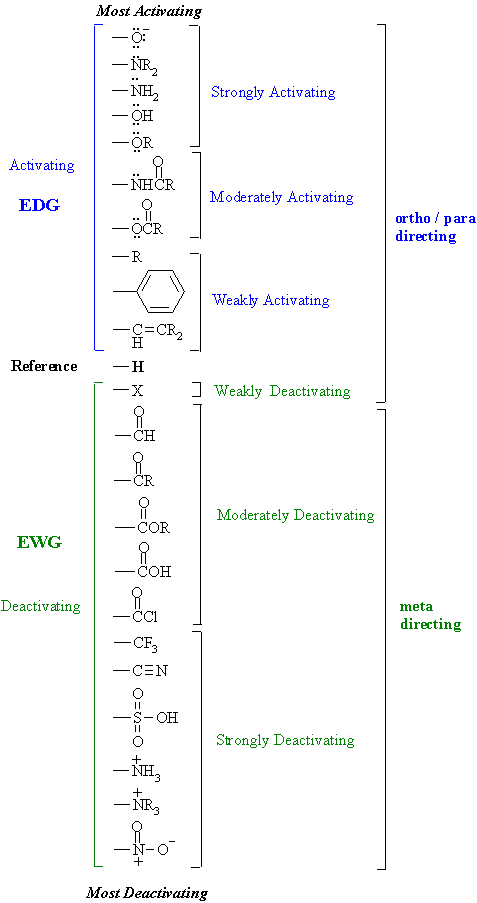 |
These effects are a combination of
RESONANCE and INDUCTIVE
effects Here are some general pointers for recognising the substituent effects:
There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the pi system and can be represented by resonance structures. These can be either electron donating (e.g. -OCH3) where pi electrons are pushed toward the arene or electron withdrawing (e.g. -C=O) where pi electrons are drawn away from the arene. In certain cases, molecules can be represent by
more than one reasonable Lewis structure that differ only in the
location of π electrons. (note that a resonance hybrid cannot normally be written as an individual Lewis diagram !). You should be able to draw all reasonable resonance structures for a given organic molecule. The best way to "derive" resonance structures is
by learning to "push"
curly arrows and starting from a reasonable
Lewis structure. INDUCTIVE effects are those that occur through the sigma system due to electronegativity effects. These too can be either electron donating electron donating (e.g. -Me) where sigma electrons are pushed toward the arene or electron withdrawing (e.g. -CF3, +NR3) where sigma electrons are drawn away from the arene.
Electronegativity
A simplified approach to
understanding substituent effects is provided, based on the "isolated
molecule approach". The text (as do most others) uses the
more rigourous approach of
drawing the resonance structures for each of the intermediate
carbocations formed by attack at each of the o-, m- and p- positions
and looking at how the initial substituent influences the stability of
the system. We are going to break down
the types of substituents into various subgroups
based on the structural features of the
substituent immediately adjacent to the aromatic ring:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
![]()